How is Produce Hot Dip Galvanized? Hot Dip Galvanized Kg Price
Hot dipped galvanized, known by the abbreviation SDG, is made to protect the steel. It is important that materials to be used in sectors such as automotive and construction do not rust for a long time. These materials are coated to resist corrosion. Surface appearance, coating thickness, mechanical properties and corrosion behavior change after zinc coating. In this way, the material is much better quality.
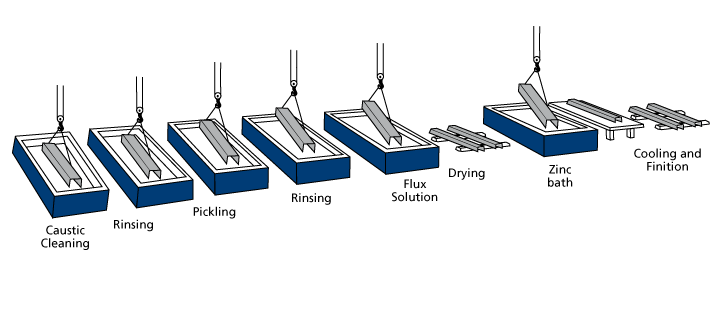
It is necessary to prepare the material before hot dip galvanizing. After the process is completed, the material that is approved by the quality control is ready to be shipped. Galvanizing process steps are as follows:
Hot Dip Galvanized?
In order for the materials to be coated in the best way, the surface must be clean. If there are unwanted residues on it, there will be roughness after coating. For this reason, surface cleaning processes are applied to the material. Chemical substances such as oil and grease on the surface of the material are difficult to acidify. In order to make this process much easier, the material is put into the degreasing bath. Thanks to this process, also called alkaline bath, the dirt layer is destroyed. Alkaline salts used in degreasing are sold in powder or crystal form. It is prepared by dissolving in water according to the instructions for use.
Surface Cleaning with Acid
If there is rust on the material, it will not be removed by degreasing. The oxide layer in the metal will prevent the iron from reacting with the zinc during galvanizing. For this reason, it is thrown into an acid bath to get rid of rust and oxide layer. Hydrochloric acid is used in surface cleaning with acid. The acid value of the pool is measured at certain intervals to ensure it remains at the desired rate. Iron immersed in the pool can interact with the acid. In order to protect the iron, an inhibitor, which is a substance that does not affect the reaction rate, is added as it enters.
The material should be removed from the pool where it was thrown in a timely manner. This stage is critical in galvanized coating. If it remains too much, abrasions may be observed on the surface. Since the rate of rust in every material is not the same, it must be attached to different hangers. It should be checked continuously throughout the application and the rust-free materials should be removed from the bath. Work at room temperature to prevent any harmful gas from the acid bath.
Rinse
The rinsing process is done to remove the hydrochloric acid remaining on the surface of the material. The material is immersed in a water-filled pool and taken out. Thus, the material is prepared for the next stage. This process takes an extremely short time, but needs to be done carefully.
Flax Bath
It is immersed in flax bath to prevent rusting of the steel surface before galvanizing. During hot galvanizing, a rapid reaction between iron and zinc is desired. Thanks to the fast reaction, a smoother surface is obtained. Flax process will also destroy residues left in the material after previous processes. Powders adhering to the material surface in the Flax bath are beneficial during galvanizing. In this way, steel and zinc react more easily.
Drying
After the flax process, the drying phase is started. This stage is important for job security. If the material is not dried, it can explode when immersed in the pool. The explosion will cause zinc depletion and environmental pollution. During the drying period, the flux solution in the material is evaporated. In this way, a thin flux layer remains on the surface.
Galvanized
Materials that pass the preparatory processes successfully and are approved by quality controllers to be suitable for galvanizing are brought to the coating area. Here, it is dipped into the zinc pool for a certain period of time and covered. How long the material will stay in the pool will be calculated depending on its weight and wall thickness.
In order for the coating to take place, the material temperature must remain in the pool until it reaches bath temperature. During the coating of the material, there is a thin oxide layer and chemicals from previous processes on the surface of the pool. The residues on the surface should be removed before the material is taken out. Thus, the quality of the coating obtained does not decrease.
Cooling-Watering
After the galvanizing process is completed, the material starts to cool rapidly after being removed from the bath up to 400 degrees. The decrease in temperature causes the zinc cover on its surface to thicken. It can be cooled in water, air or oil. If cooling is to be made in air, the environment should be free of dust. Otherwise, this application will not work well. Cooling in water provides very fast cooling. In this case, roughness occurs on the surface. If a very smooth surface is desired, water cooling should not be done. However, partial water cooling can be applied by cooling the part with both water and air. In this case, the inner and outer surfaces of the piece will have a shiny appearance.
The purpose of quenching is to ensure better adhesion of the coating to the material. The coating will be smoother if some soap and oil are added to the water. Corrosive salts may come from the flux layer into the water used, so it should be changed frequently.
Hot Dip Galvanized Kg Price
Hot dip galvanized kg price is determined depending on the type of material and the processes to be applied. There may be fluctuations in prices depending on the movements in the exchange rate and the market demand. You can get the price per kilogram according to the thickness of your material. If you want to cover a high percentage of material, the kilogram price will be more appropriate. You can contact us for information about the price.